www.fluorescencemicroscopy.it
Main menu:
- Home Page
- Microscopes
- Fluorescence
- Description
- Diascopy
- Epi-Fluoresc.
- Illumination
- Fluorocromes
- The Fading
- Wavelengths
- Properties
- % of transmission
- Types of filters
- Overlapping Spectra
- Intensity
- Image acquisition
- The Filter's set
- The Filters
- Fluorochrome's praparation
- Sample's preparations
- Fluorescence Sample
- Cytochemical Fluorescence
- Intrinsic Fluorescence
- Gallery
- Phase Contrast
- Polarization
- Darkfield
- DIC
- COL
- Rheinberg
- Brightfield
Wavelengths
Fluorescence
The Wavelengths
Any portion of the electromagnetic spectrum is qualitatively defined by the wavelength of light that composes it. In other words, the wavelength of a light wave tells us almost everything we want to know. The nanometer (nm) is the unit most commonly used in Fluorescence microscopy, and the additional spectrum that is typically reported as perceived by humans ranges from about 380 nm to 710nm. Although varying from subject to subject and therefore difficult to quantify, only about 15% of humans can perceive over 680nm and is not recommended see below 420nm due to the risk of eye damage. The visible spectrum can be divided into intervals of particular wavelength, each of which corresponds to what we call color:
Purple/Indigo |
380 - 450 nm |
Blue/Cyano |
450 - 500 nm |
Green |
500 - 570 nm |
Yellow/Orange |
570 - 610 nm |
Red |
610 - 710 nm |
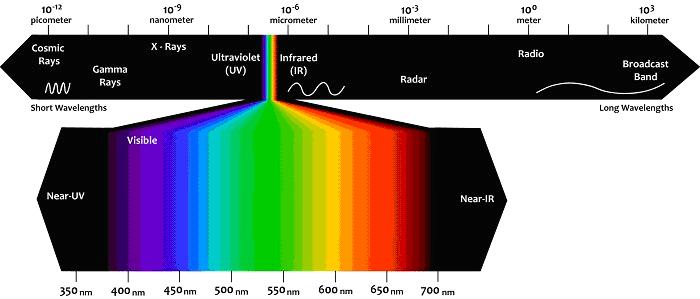
Figure 1: View of the electromagnetic spectrum, which is quantified using the wavelengths,
with the visible region highlighted.
Also the wavelengths at limits of the visible spectrum are used in fluorescence. They include short wavelength band to 320-400 nm (near UV) and long-band wavelengths from 750 to about 2500 nm (near IR).
In the optical, the wavelength (and therefore the frequency) is also directly proportional to the energy of a wave, as originally described by Max Planck with the following equation:
E = hc / ë
Where E is energy, h is Planck's constant, c is the speed of light, and ëis the wavelength of light. This report expresses the idea that the light of shorter wavelength (ie purple) has more energy than light of longer wavelengths (ie red).
Sub-Menu:
- Description
- Diascopy
- Epi-Fluoresc.
- Illumination
- Fluorocromes
- The Fading
- Wavelengths ←
- Properties
- % of transmission
- Types of filters
- Overlapping Spectra
- Intensity
- Image acquisition
- The Filter's set
- The Filters
- Fluorochrome's praparation
- Sample's preparations
- Fluorescence Sample
- Cytochemical Fluorescence
- Intrinsic Fluorescence
- Gallery