www.fluorescencemicroscopy.it
Main menu:
- Home Page
- Microscopes
- Fluorescence
- Description
- Diascopy
- Epi-Fluoresc.
- Illumination
- Installing and Aligning a Mercury Lamp
- Fluorocromes
- Tables of Fluorocromes
- The Fading
- Wavelengths
- Properties
- % of transmission
- Types of filters
- Overlapping Spectra
- Intensity
- Image acquisition
- The Filter's set
- The Filters
- Fluorochrome's praparation
- Sample's preparations
- Fluorescence Sample
- Cytochemical Fluorescence
- Intrinsic Fluorescence
- Gallery
- Phase Contrast
- Polarization
- Darkfield
- DIC
- COL
- Rheinberg
- Brightfield
The Fading
Fluorescence
Fading, quenching, e photobleaching
A wide range of conditions and factors often come into play to affect the phenomena of excitation and emission of fluorescence and hence reduce the intensity.
The general term for a reduction of fluorescence intensity is fading, a term that brings together the phenomena of quenching and photobleaching.
The two phenomena are distinguished by the fact that the quenching is often reversible whereas photobleaching is not.
For photobleaching means irreversible decomposition of the fluorescent molecules in the excited state due to their interaction with oxygen molecules before emission process.
The rate of photobleaching depends on several factors, including the chemical reactivity of the fluorochrome, the intracellular chemical environment, the intensity and wavelength of excitation light. Some fluorescent dyes are easily susceptible to photobleaching while others are relatively stable. In some cases, the sample may recover from the effects of photobleaching, especially if it is kept in a cool, dark environment. The phenomenon of photobleaching can be minimized by reducing the exposure time or decreasing the excitation energy (lamp intensity), neutral density filters can be inserted, in order, in the light path before it reaches the excitation filter, thus decreasing the intensity of the light. However, these remedies are accompanied undesirable effect of reducing the intensity emission of the fluorophore. It's also useful, where possible, the use of specific substances "antifade" currently available commercially. The phenomenon of fading can also be reduced, in some cases, changing the concentration of the pH of the mounting medium. Another method is to increase the concentration of the fluorophore or the use of more appropriate fluorochromes less susceptible to photobleaching.
The occurrence of photobleaching is exploited in a technique known as fluorescence recovery after photobleaching (FRAP), a very useful mechanism to investigate the distribution and movement of biological macromolecules. The method is based on the photobleaching of a well-defined region of the sample exposed to intense laser light. Then, the rates and the recovery mode of fluorescence in the area are analyzed. A related technique, known as the fluorescence loss in photobleaching (FLIP), is used to monitor the decrease in fluorescence in a region adjacent to where photobleaching occurred. Similar to FRAP, the latter technique is useful for studying the molecular mobility and dynamics of living cells.
Photobleaching of the phenomenon must be distinguished from another artifact of fluorescence, the quenching, which occurs through the reduction (or in some cases, the enhancement) of fluorescence intensity due to competing processes such as temperature, high concentrations of oxygen, and the molecular aggregation in the presence of salts or halogen compounds. A common example of quenching was observed by a collision of the fluorophore excited state with another molecule in solution (not fluorescent), and the result is the deactivation of the fluorophore and the return to the ground state. In most cases, none of the molecules is chemically modified in the process of quenching due to collision. Most quenching processes involved reducing the length of the excited state and the quantum yield of the fluorophore.
Sometimes the quenching is the result of transfer of energy to other acceptor molecules that reside physically close to the excited fluorophore, a phenomenon known as resonance energy transfer. This particular phenomenon has become the basis for a more recent technique to measure distances much below the lateral resolution of optical microscopy (FRET). Even the impurities of fluorochromes can help reduce the intensity of fluorescence photobleaching or causing quencing.
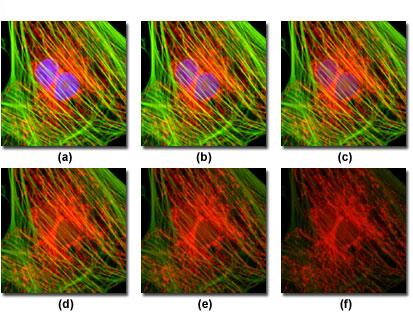
The figure above describes a typical example of photobleaching (fading) observed in a series of digital images captured at different times relative to a culture of cells.
The nuclei were stained with DAPI (blue fluorescence) and the actin cytoskeleton and mitochondria, respectively, were stained with MitoTracker Red (red fluorescence) and Alexa Fluor 488 (green fluorescence). The photos were taken at intervals of two minutes with a combination of fluorescence filters with bandwidths selected to excite the three fluorophores simultaneously (tri-band filters). Note that all three fluorophores have a relatively high intensity under (a), but the relative intensity in DAPI (blue) starts to drop rapidly at two minutes and is almost completely sold out in six minutes. The mitochondrial and actin stains are more resistant to photobleaching, but the intensity of both diminishes greatly over the timeline (10 minutes). The registration of fluorescence events in this context, it becomes problematic, a solution to limit the problem is to increase the detection sensitivity of the means of recovery, using digital cameras with "low-light level" CCD, specifically designed for microscopy fluorescence.
Sub-Menu:
- Description
- Diascopy
- Epi-Fluoresc.
- Illumination
- Installing and Aligning a Mercury Lamp
- Fluorocromes
- Tables of Fluorocromes
- The Fading ←
- Wavelengths
- Properties
- % of transmission
- Types of filters
- Overlapping Spectra
- Intensity
- Image acquisition
- The Filter's set
- The Filters
- Fluorochrome's praparation
- Sample's preparations
- Fluorescence Sample
- Cytochemical Fluorescence
- Intrinsic Fluorescence
- Gallery